Discovery of the Naturally Occurring Pure CaTiO3 Cubic Perovskite from Tazheran Massif
1. State Key Laboratory of Petroleum Resources and Prospecting, China University of Petroleum, Beijing, 102249, China
2. College of Geoscience, China University of Petroleum, Beijing, 102249, China
Jin Liu
Center for High Pressure Science and Technology Advanced Research, Beijing, 102249, China
Fuyang Liu
Center for High Pressure Science and Technology Advanced Research, Beijing, 102249, China
Eugene Sklyarov
Institute of the Earth's Crust, Siberian Branch of the Russian Academy of Sciences, Irkutsk 664033, Russia
Ross N. Mitchell
Institute of Geology and Geophysics, Chinese Academy of Sciences, Beijing, 100029, China
DOI: https://doi.org/10.36956/eps.v3i1.991
Received: 23 November 2023; Received in revised form: 14 March 2024; Accepted: 7 April 2024; Published: 25 April 2024
Copyright © 2024 Author(s). Published by Nan Yang Academy of Sciences Pte. Ltd.
 This is an open access article under the Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0) License.
This is an open access article under the Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0) License.
Abstract
As the parent compound of the perovskite-structured family and an analogue of davemaoite CaSiO3 in Earth’s lower mantle, CaTiO3 perovskite is widely used in electronic ceramic materials, immobilizing radioactive waste, and geosciences. Here we report the discovery of a natural pure CaTiO3 perovskite-C in calc-silicate veins in brucite marble in the Tazheran Massif, Russia. The mean composition of perovskite-C is 97 wt% total CaO and TiO2, and minor impurities of Na, Al, and Zr, yielding the chemical formula CaTi0.97Al0.01Na0.01Zr0.01O3. The cubic crystal structure—Pm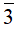 m, Z = 1: a = 3.8301 Å, V = 56.19 (2) Å3 (ρ = 4.019 g/cm3) has been refined to R1 = 0.0451. Compared with the cubic perovskite discovered in Israel, the cubic perovskite discovered in this study is very pure in Ca and Ti. The naturally occurring perovskite-C from Tazheran implies that the host skarn might have experienced high-temperature (> 1247–1374 °C) metamorphism and/or metasomatism at an early stage. Moreover, the discovery of natural perovskite-C also draws attention to considering crystal-structure-related matrix effects in the future of in situ U-Pb geochronology, especially using Tazh international perovskite standards.
m, Z = 1: a = 3.8301 Å, V = 56.19 (2) Å3 (ρ = 4.019 g/cm3) has been refined to R1 = 0.0451. Compared with the cubic perovskite discovered in Israel, the cubic perovskite discovered in this study is very pure in Ca and Ti. The naturally occurring perovskite-C from Tazheran implies that the host skarn might have experienced high-temperature (> 1247–1374 °C) metamorphism and/or metasomatism at an early stage. Moreover, the discovery of natural perovskite-C also draws attention to considering crystal-structure-related matrix effects in the future of in situ U-Pb geochronology, especially using Tazh international perovskite standards.
Keywords: Perovskite; Cubic crystal structure; CaTiO3; SCXRD; Tazheran Massif
References
[1] Rose, G., 1839. Description of some new minerals from the Urals. Pogendorff Annals of Physics and Chemistry. 48, 551–572. (in German). DOI: https://doi.org/10.1002/andp.18391241205
[2] Ali, R., Yashima, M., 2005. Space group and crystal structure of the perovskite CaTiO3 from 296 to 1720K. Journal of Solid State Chemistry. 178(9), 2867–2872. DOI: https://doi.org/10.1016/j.jssc.2005.06.027
[3] Ball, C.J., Begg, B.D., Cookson, D.J., et al., 1998. Structures in the system CaTiO3/SrTiO3. Journal of Solid State Chemistry. 139(2), 238–247. DOI: https://doi.org/10.1006/jssc.1998.7836
[4] Newnham, R.E., Ruschau, G.R., 1991. Smart electroceramics. Journal of the American Chemical Society. 74, 463–480. DOI: https://doi.org/10.1111/j.1151-2916.1991.tb04047.x
[5] Wang, R.C., Xu, S.J., Lu, J.J., et al., 2000. Crystal-chemistry and geochemistry of perovskite-group minerals. Earth Science Frontiers. 7(2), 457–465. (in Chinese).
[6] Harte, B., Hudson, N.F.C. (editors), 2013. Mineral associations in diamonds from the lowermost upper mantle and uppermost lower mantle. Proceedings of the 10th International Kimberlite Conference; 2013 Jan 1st; Bangalore, India. p 235–253. DOI: https://doi.org/10.1007/978-81-322-1170-9_15
[7] Nestola, F., Korolev, N., Kopylova, M., et al., 2018. CaSiO3 perovskite in diamond indicates the recycling of oceanic crust into the lower mantle. Nature. 555, 237–241. DOI: https://doi.org/10.1038/nature25972
[8] Ringwood, A.E., 1962. A Model for the upper mantle: 2. Journal of Geophysical Research. 67(11), 4473–4478. DOI: https://doi.org/10.1029/JZ067i011p04473
[9] Glazer, A.M., 1972. The classification of tilted octahedra in perovskites. Acta Crystallographica. B28, 3384–3392. DOI: https://doi.org/10.1107/S0567740872007976
[10] Howard, C.J., Stokes, H.T., 1998. Group theoretical analysis of octahedral tilting in perovskites. Acta Crystallographica. B54, 782–789. DOI: https://doi.org/10.1107/S0108768198004200
[11] Howard, C.J., Stokes, H.T., 2005. Structures and phase transitions in perovskites—A group theoretical approach. Acta Crystallographica. A61, 93–111. DOI: https://doi.org/10.1107/S0108767304024493
[12] Mitchell, R.H., 2002. Perovskites: Modern and Ancient. Almaz Press Inc.: Thunder Bay, ON, Canada. pp. 318.
[13] Mitchell, R.H., Burns, P.C., Chakhmouradian, A.R., 2000. The crystal structures of loparite-(Ce). The Canadian Mineralogist. 38(1), 145–152. DOI: https://doi.org/10.2113/gscanmin.38.1.145
[14] Mitchell, R.H., Welch, M.D., Chakhmouradian, A.R., 2017. Nomenclature of the perovskite super group: A hierarchical system of classification based on crystal structure and composition. Mineralogical Magazine. 81(3), 411–461. DOI: https://doi.org/10.1180/minmag.2016.080.156
[15] Kennedy, B.J., Howard, C.J., Chakoumakos, B.C., 1999. Phase transitions in perovskite at elevated temperatures—a powder neutron diffraction study. Journal of Physics: Condensed Matter. 11, 1479–1488. DOI: https://doi.org/10.1088/0953-8984/11/6/012
[16] Redfern, S.A.T., 1996. High-temperature structural phase transitions in perovskite (CaTiO3). Journal of Physics: Condensed Matter. 8, 8267–8275. DOI: https://doi.org/10.1088/0953-8984/8/43/019
[17] Vogt, T., Schmahl, W.W., 1993. The high-temperature phase transition in perovskite. Europhysics Letters. 24(4), 281–285. DOI: https://doi.org/10.1209/0295-5075/24/4/008
[18] Wang, Y., Liebermann, R.C., 1993. Electron microscopy study of domain structure due to phase transitions in natural perovskite. Physics and Chemistry of Minerals. 20, 147–158. DOI: https://doi.org/10.1007/BF00200117
[19] Barth, T., 1925. The crystal structure of perovskite and related compounds. Norsk Geologisk Tidsskrift. 8, 201–216. Available from: https://njg.geologi.no/publications/die-kristallstruktur-von-perowskit-und-verwandten-verbindungen/ (in German).
[20] Britvin, S.N., Vlasenko, N.S., Aslandukov, A., et al., 2022. Natural cubic perovskite, Ca(Ti,Si,Cr)O3-δ, a versatile potential host for rock-forming and less-common elements up to Earth’s mantle pressure. American Mineralogist. 107(10), 1936–1945. DOI: https://doi.org/10.2138/am-2022-8186
[21] Sklyarov, E.V., Karmanov, N.S., Lavrenchuk, A.V., et al., 2019. Perovskites of the Tazheran Massif (Baikal, Russia). Minerals. 9(5), 323. DOI: https://doi.org/10.3390/min9050323
[22] Sun, J., Wu, F.Y., Sklyarov, E., et al., 2022. Matrix effects during in situ U-Pb dating of perovskite with variable crystal structure: Evidence from the Tazheran Massif, Russia. Chemical Geology. 589, 120685. DOI: https://doi.org/10.1016/j.chemgeo.2021.120685
[23] Armstrong, J.T., 1995. CITZAF-A package of correction programs for the quantitative electron microbeam X-ray-analysis of thick polished materials, thin-films, and particles. Microbeam Analysis. 4(3), 177–200.
[24] Gautheron, C., Hueck, M., Ternois, S., et al., 2022. Investigating the shallow to mid-depth (>100–300 °C) continental crust evolution with (U-Th)/He thermochronology: a review. Minerals. 12(5), 563. DOI: https://doi.org/10.3390/min12050563
[25] Magennis, S.W., Ferguson, A.J., Bryden, T., et al., 2003. Time-dependence of erbium(III) tris(8-hydroxyquinolate) near-infrared photoluminescence: implications for organic light-emitting diode efficiency. Synthetic Metals. 138(3), 463–469. DOI: https://doi.org/10.1016/S0379-6779(02)00501-5
[26] Doroshkevich, A., Sklyarov, E., Starikova, A., et al., 2017. Stable isotope (C, O, H) characteristics and genesis of the Tazheran brucite marbles and skarns, Olkhon region, Russia. Mineralogy and Petrology. 111, 399–416. DOI: https://doi.org/10.1007/s00710-016-0477-8
[27] Meinert, L.D., Dipple, G.M., Nicolescu, S., 2005. World Skarn Deposits. Society of Economic Geologists, Inc. Economic Geology 100th Anniversary. 299–336. DOI: https://doi.org/10.5382/AV100.11
[28] Wallmach, T., Hatton, C.J., 1989. Extreme facies of contact metamorphism developed in calc-silicate xenoliths in the eastern Bushveld Complex. Canadian Mineralogist. 27(3), 509–523.
[29] Qin, S., Wang, R.C., 2004. Geometric descriptions of distorted structures of ABX3 type perovskite and application in structural prediction. Acta Geological Sinica. 78(3), 345–351. Available from: https://www.geojournals.cn/dzxb/dzxb/article/abstract/20040345 (in Chinese).
[30] Thomas, N.W., 1998. A new global parameterization of perovskite structures. Acta Crystallographica. B54, 585–599. DOI: https://doi.org/10.1107/S0108768198001979
[31] Black, L.P., Kamo, S.L., Allen, C.M., et al., 2004. Improved 206Pb/238U microprobe geochronology by the monitoring of a trace-element-related matrix effect; SHRIMP, ID-TIMS, ELA-ICP-MS and oxygen isotope documentation for a series of zircon standards. Chemical Geology. 205(1–2), 115–140. DOI: https://doi.org/10.1016/j.chemgeo.2004.01.003
[32] Finch, R.J., Hanchar, J.M., Hoskin P.W.O., 2001. Rare-earth elements in synthetic zircon: Part 2. A single-crystal X-ray study of xenotime substitution. American Mineralogist. 86(5–6), 681–689. DOI: https://doi.org/10.2138/am-2001-5-608
[33] Pohlner, J.E., Schmitt, A.K., Chamberlain, K.R., et al., 2020. Baddeleyite microtextures and U-Pb discordance: insights from the Spread Eagle Intrusive Complex and Cape St. Mary’s sills, Newfoundland, Canada. Geochronology Discussions. DOI: https://doi.org/10.5194/gchron-2019-21
[34] Wingate, M.T.D., Compston, W., 2000. Crystal orientation effects during ion microprobe U–Pb analysis of baddeleyite. Chemical Geology. 168(1–2), 75–97. DOI: https://doi.org/10.1016/S0009-2541(00)00184-4

